A galvanic or voltaic cell is a redox reaction that produces electricity. The following diagram shows a Daniell cell that uses the Zn/Cu²⁺ reaction. This reaction may be separated out so that you have an indirect electron transfer and can produce some useable electricity.
Galvanic cells are commonly called batteries, but sometimes this name is somewhat incorrect. A battery is composed of two or more cells connected together. You put a battery in your car, but you put a cell into your flashlight.
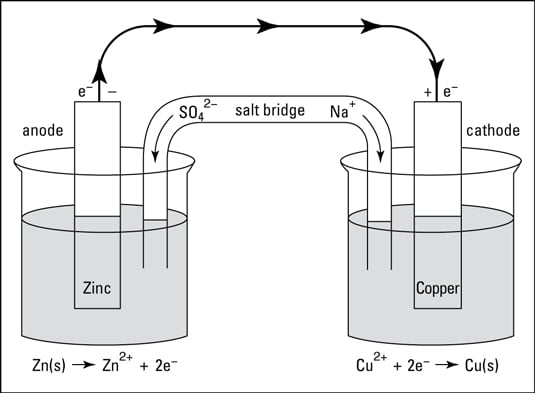
The electrodes act as a terminal, or a holding place, for electrons. A wire connects the electrodes, but nothing happens until you put a salt bridge between the two containers. The salt bridge, normally a U-shaped hollow tube filled with a concentrated salt solution, provides a way for ions to move from one container to the other to keep the solutions electrically neutral.
With the salt bridge in place, electrons can start to flow. Zinc is being oxidized, releasing electrons that flow through the wire to the copper electrode, where they’re available for the Cu²⁺ ions to use in forming copper metal. Copper ions from the copper(II) sulfate solution are being plated out on the copper electrode, while the zinc electrode is being consumed.
The cations in the salt bridge migrate to the container containing the copper electrode to replace the copper ions being consumed, while the anions in the salt bridge migrate toward the zinc side, where they keep the solution containing the newly formed Zn²⁺ cations electrically neutral.
The zinc electrode is called the anode, the electrode at which oxidation takes place, and is labeled with a “–” sign. The copper electrode is called the cathode, the electrode at which reduction takes place, and is labeled with a “+” sign.
This cell will produce a little over one volt. You can get just a little more voltage if you make the solutions that the electrodes are in very concentrated.

